- Here solid NaCl is mixed with water to form an aqueous solution. Which of the following reactions would result in increased entropy.
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic
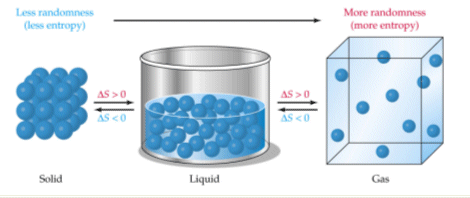
This results in an increase in entropy.

. H 601 kJmol 258 JK The following solution may contain one or more values that are different from the problem provided to you however the steps to solve the problem are the same. 2 question Which of the following reactions would result in decreased entropy. When the piece of a jigsaw puzzle space dumped native the box the.
Hence randomness is decreasing here. 2039 3029 O D. Which of the following reactions would result in decreased entropy.
CO2 s CO2 9 O C. H20 g H20 1 O B. H20g H201 O B.
CO2s CO29 O C. The reaction that would result in decreased entropy is N2 3H2 2NH3. 2039 3029 O D.
The reaction would result in an increase in entropy S and a decrease in the energy content H of the system. Which statement best defines specific heat. CO2s CO29 O C.
N2 g 3H2 g - 2NH3 g - Apex Correct answer if youre taking the Unit 6 Exam joonation. Leave a Reply Cancel reply. Which of the following would result in decreased entropy.
Hence entropy is increasing. B ice melting hope it helps. The amount of heat required to.
What is the entropy when 129. H20g H201 O B. The reaction would result in products with a greater free.
2039 Which of the following reactions would result in decreased entropy. You might be interested in. On apex it is water freezing.
CO2s CO29 O C. What will certainly it look at like. Which of the following reactions would result in decreased entropy.
H20g H201 O B. The reaction would result in a decrease in entropy S and an increase in the energy content H of the system. Which of the following reactions would result in decreased entropy The entropy of a system increases whenever its particles have more freedom of motion.
Which of the following reactions would result in decreased entropy. Inessss 21 1 year ago. Determine S for the phase change of 117 moles of water from solid to liquid at 0C.
N22049 2NO39 Categories English. H20g H201 O B. Maria 59 1 year ago.
CO2s CO29 O C. CO2s CO29 O C. Helpful 5 Not Helpful 0 Add a Comment.
Leave a Reply Cancel reply. A Preparing aqueous solution of common salt. 2039 3029 O D.
2039 3029 O D. B Change of water into ice- matter in solid state are more organized. The reaction could be coupled to power an endergonic reaction with a G of 88 kcalmol.
CO2s CO29 O C. Thus the entropy increases whenever girlfriend have an ext moles that gaseous commodities than of reactants and whenever girlfriend have an ext product corpuscle in solution than you have of. KNO3 s K aq NO3- aq Wiki User.
Your email address will not be published. CO2s CO29 O C. Correct answer to the question Which of the following reactions would result in decreased entropy.
H20g H201 O B. 2039 Which of the following reactions would result in decreased entropy. H20g H201 O B.
Lets understand the options in an easy way. Your email address will not be published. Which of the following reactions would result in decreased entropy.
N22049 2NO39 Categories English. 2039 3029 O D. So randomness is increasing.
H20g H201 O B. First calculate the heat for 110 moles. Define entropyList determinants that influence the entropy the a systemList situations that show the ide of entropy.
Which of the following reactions would result in increased entropy.

Which Of The Following Reaction Would Result In A Decrease In Entropy Brainly Com

Which Of The Following Reactions Would Result In Decreased Entropy Multiple Choice Brainly Com

0 Comments